

VESPR stands for valence shell electron pair repulsion.

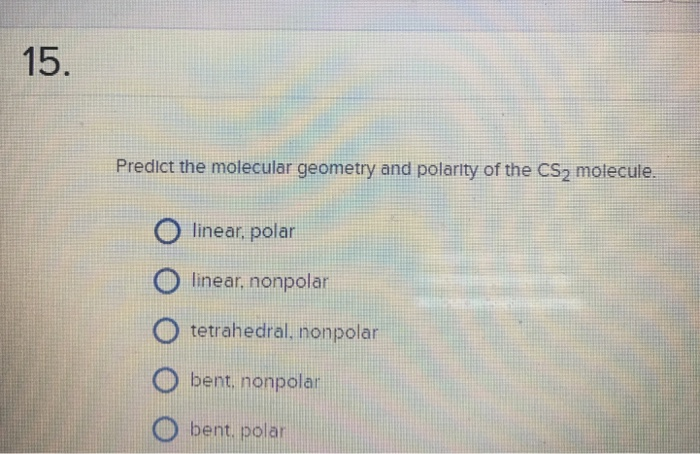
It applies a theory called VESPR for short. Determine the electron group arrangement around the central atom that minimizes repulsions. 1)The molecular geometry of the CS2 molecule is. Molecular geometry is a way of describing the shapes of molecules.Draw the Lewis electron structure of the molecule or polyatomic ion.This VESPR procedure is summarized as follows: VESPR Produce to predict Molecular geometry Using this information, we can describe the molecular geometry, the arrangement of the bonded atoms in a molecule or polyatomic ion. From the BP and LP interactions we can predict both the relative positions of the atoms and the angles between the bonds, called the bond angles. a) An atom with 4 electron containing regions, two of which are covalent bonds. Each group around the central atom is designated as a bonding pair (BP) or lone (nonbonding) pair (LP). Hence, the bonded Sulphur atoms are arranged linearly around the central Carbon atom. In the CS2 lewis structure, there is a total of 4 lone pairs present. CS2 has 2 electron domains, resulting in a linear electron domain geometry. In the VSEPR model, the molecule or polyatomic ion is given an AX mE n designation, where A is the central atom, X is a bonded atom, E is a nonbonding valence electron group (usually a lone pair of electrons), and m and n are integers. Molecular geometry for CS2 The molecular geometry of CS2 is linear because the central atom Carbon is bonded to the two Sulphur bonds and it contains no lone pair of electrons to cause molecular distortion. It has a linear geometry arrangement like SCS. So, there are three regions of electron density around the carbon central atom. The smallest particles which have complete properties of. The molecular geometry of CH2O is trigonal planar as the carbon central atom has no lone pair and is attached to the two hydrogens atoms and one oxygen atom with the help of two single bonds and one double bond.

Groups are placed around the central atom in a way that produces a molecular structure with the lowest energy, that is, the one that minimizes repulsions. Carbon disulphide is an organic compound or molecule made up of one carbon and two sulphur atoms. If there is no lone pair present in the central atom, then the electron pair arrangement and molecular geometry would be the same.\): Electron Geometries for Species with Two to Six Electron Groups. (a) CF4 - tetrahedral: (b) BeBr2 - linear: (c) H2O. We have to know that if there is a lone pair present in the central atom, then the electron pair arrangement and molecular geometry would be different. Choose the molecule that is incorrectly matched with the electronic geometry about the central atom. $Steric\ Number = No.\ of\ bonded\ pair\sin central\ atom + Number\ of\ lone\ pairs\ present\ in\ central\ atom$ $$ molecule is linear. shape and polarity for each of the following molecules : a ) CS2 b ) SO3. We can see that two atoms of sulfur are bonded to the central carbon atom, so the number of bonded pairs in the central atom is two. An Introduction to Atomic and Molecular Structure Harry B Gray, Harry B. $Steric\ Number = No.\ of\ bonded\ pair\sin central\ atom + Number\ Lone\ pairs\ present\ in\ central\ atom$ From the Lewis structure, let us now calculate the steric number. We can write the formula of steric number as,


 0 kommentar(er)
0 kommentar(er)
